One would think that the common aquarium fish should have no
problems concerning their proper scientific names, especially if
they have been in the hobby for many years. But in many of the
popular aquarium fishes a name is generally applied, usually by
a scientist, which is immediately accepted by all concerned, and
no further inquiry is made. And after all, since they are
common, specimens should be readily available for investigation
by scientists in case some question arises as to their proper
identity.
Unfortunately this is not always the case. Local breeders
successful in propagating the initially high-priced imports, and
domestic stocks soon are plentiful enough at low prices to
satisfy most of the needs of aquarists. The imports naturally
drop off considerably as domestic supplies increase and
eventually stop altogether. In addition, easy-to-breed species
that are genetically variable are selectively bred to produce
any number of strains often quite different in appearance from
the natural species. Wild-caught specimens are therefore not as
available through the aquarium trade as one would think. Any
inquiries into the systematic problems of such fishes would
necessitate visits to museums where collections of the species
concerned are held or travel to their natural habitats (as was
done by Dr. Herbert R. Axelrod). The common angelfishes of the
genus Pterophyllum seem to be one of these groups that has
caused a great deal of trouble.
The first angelfish ever described was a species called Zeus scalaris by Lichtenstein in 1823. It was collected in “Brazil”
and apparently deposited in the Berlin Museum. Cuvier (or
Valenciennes) found the specimen there labeled Zeus scalaris and
was probably unaware that Lichtenstein had already published its
description. They therefore described it as new, kept the
specific name scalaris, but decided that it was in the wrong
genus, naming it Platax? scalaris. They apparently were not
satisfied with placing it in the genus Platax (hence the
question mark) and thought that when it became better known (the
specimen was in a “mutilated” condition and only an incomplete
description could be given) a new genus could be erected for it.
This was apparently accomplished some nine years later (in 1840)
by Heckel, who was able to see additional specimens from the Rio
Negro. He called the new genus Pterophyllum (meaning fins like
leaves), which included at that time the single species Pterophyllum scalaris. In 1855 Castelnau described a new genus
and species of this same type fish, calling it Plataxoides
dumerilii, seemingly unaware of the fish described by Cuvier and
Valenciennes. Guenther, in his Catalogue of the Fishes in the
British Museum (Natural History), simplified everything by synonymizing
Plataxoides with Pterophyllum and placing all the
species thus far described (Platax scalaris, Pterophyllum
scalaris, and Plataxoides dumerilii, but not Zeus scalaris of
which he was probably unaware) under the slightly modified name
Pterophyllum scalare. There were then either one (P. scalare) or
two (P. scalare and P. dumerilii) species of angelfishes
depending upon whether one agreed with Guenther or not. At least
they were finally placed together in a single genus.
In 1903 another angelfish species, Pterophyllum altum, was
described by Pellegrin, and in 1928 a fourth species, P. eimekei
Ahl, was added to the list. The most recent species described,
Plataxoides leopoldi Gosse, was placed in Castelnau’s genus
because Gosse believed that the name Pterophyllum was
preoccupied by a genus of insects called Pterophyllum Harris.
But Schultz, in reviewing the genus Pterophyllum in 1967,
checked on the name and found that Harris consistently used the
name Pterophylla, not Pterophyllum as erroneously reported. The
genus name Pterophyllum was therefore free to be used (or
available according to the terminology of the International
Rules of Nomenclature).
At this point in time there were five
named or nominal species: Pterophyllum scalare, P. dumerilii, P.
altum, P. eimekei and P. leopoldi. But how many real species of
angel-fishes were there?
It seems that Schultz was able to examine the holotype of P. dumerilii and the paratypes of
P. leopoldi and concluded that
they are one and the same species. Comparisons of P. scalare
specimens with those of P. eimekei led Schultz to consider the
latter species a synonym of the former. The remaining species,
P. altum, he considered valid although casting some doubt on
this decision with his statement "Undoubtedly P. altum represents the P. scalare
type of angelfish in the upper
Orinoco, and in having a higher average number of dorsal, anal,
oblique scale rows, and vertebrae than P. scalare, it might be
considered to represent only a subspecies of P. scalare;
however, since P. altum has been taken so far only in the upper
Orinoco basin, I prefer tentatively to recognize it as a
distinct species.
In recent collections by Dr. Herbert R. Axelrod in the Rio
Negro (see “The Heavenly Paradox”), some very interesting and,
as it turns out, very important specimens of angelfishes were
taken.* According to Schultz’ distributional map, the collecting
sites for which he had data on the angelfishes were restricted
to the Amazon River from its mouth to Manaus and then along the
Solimoes (Amazonas) branch of the river to Peru. There were no
collections reported on by him from the Rio Negro, which winds
its way northwestward toward the Orinoco where Pterophyllum
altum comes from! These specimens from the Rio Negro thus filled
a very important gap. The question as to whether they would show
(1) a close association with the Amazon and Solimoes forms and
be as different from P. altum as they were, (2) a close
association with the Orinoco forms and be different from the
Amazon and associated forms, or (3) would they show a continuous
gradient or cline from Manaus to the Orinoco, indicating that P. altum was really not very different after all, could at last be
answered. According to Schultz, the differences between P. altum
and P. scalare are the greater average number of soft dorsal and
anal fin rays as well as the greater average number of oblique
scale rows and vertebrae of P. altum. The color patterns were
said to be identical and therefore of no use in distinguishing
the two. Proportional measurements were made but considered
unreliable due to the great variability “even at nearly equal
sizes,” although photos of P. altum always seemed to show a fish
with more elongate dorsal and anal fins.
*Because of Dr. Axelrod's report on the short anal fin, I
examined the rays carefully under a microscope. It seems fairly
certain that the trailing anal rays and sometimes the caudal and
pelvic rays were torn or bittern off and have started to
regenerate. When I explained this to Dr. Axelrod he surmised
that the exceptionally high waters conjugated the habitats of
the angelfish with those of the piranhas, thus enabling the
piranhas to bit off their long trailing anal fins. Normally the
two habitats are distinct and these physical anomalies are not
apparent.
When the average number of dorsal and anal fin rays and oblique
scale rows were plotted on a map of the Amazon system, it was
found that Belem specimens had an average of 24.9 dorsal fin
rays, 27.5 anal fin rays and 36.7 oblique scale rows. The
average numbers dropped as one headed up-river to Porto do Moz
and Santarem (to 23.3 and 22.6 dorsal fin rays, 25.3 and 24.9
anal fin rays and 33.2 and 33.1 oblique scale rows respectively)
but increased again (to 24.6, 26.8, and 35.4) by the time one
reached Manaus. At this point the branching off of the Rio Negro
occurs. If one follows the Solimoes (Amazonas) the counts stay
about the same at least as far as Tefe (24.6, 27.1, and 35.5)
but start to drop again upstream at Tonantins (23.6, 24.9, and
33.1). With the new specimens available it was discovered that
if one follows the Rio Negro from Manaus, the counts increase
with distance from the Amazon so that at Igarape Anapichi and
Igarape Apania in the upper Rio Negro the average soft dorsal
ray count for nine individuals was 26.2, the average anal fin
soft ray count 28.3 and the average number of oblique scale rows
38.1. The identification of these specimens as either P. scalare
or P. altum on the basis of counts was not immediately obvious,
although they seemed to favor the latter species. Another group
of six specimens collected further up the Rio Negro fell nicely
into this sequence, with an average number of soft dorsal fin
rays of 28.0, anal fin rays 29.8 and oblique scale rows 40.3.*
When compared to the counts for P. altum from the Orinoco (28.6,
29.7 and 42.6 respectively) it could be seen that they were
extremely close. The Rio Negro angelfishes appear to bridge the
gap between the Orinoco populations.
*The counts that appeared in position 2 on the map appearing
in T.F.H. Magazine for January 1976, p. 95, were in error.
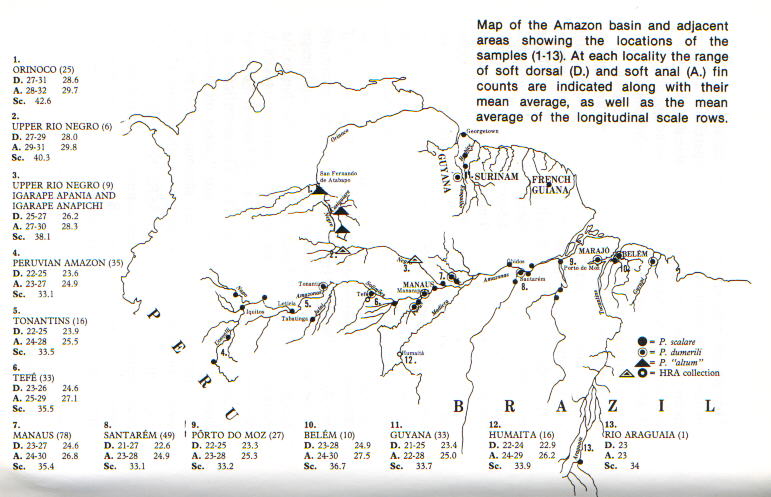
Fig.
1. Map of the Amazon basin and
adjacent areas showing the
locations of the samples (1-13).
|
| Fig. 1.
Map of the Amazon basin and adjacent areas
showing the locations of the samples (1-13). |
Map of the Amazon basin and adjacent areas showing the locations
of the samples (1-13). At each locality the range of soft dorsal
(D.) and soft anal (A.) fin counts are indicated along with
their mean average, as well as the mean average of the
longitudinal scale rows.
When one looks at the color pattern of these fish very
little can be seen to separate them at first glance. On closer
examination, however, certain distinctions become evident. If
one were again to compare the extremes (Rio Orinoco with the
Amazon or Guyana specimens) one would observe that the Amazon
and Guyana specimens possess a dark band that extends from the
chest (where it is joined by the corresponding opposite band)
through the eye to the first spines of the dorsal fin. The
Orinoco forms have a similar band that starts from the same spot
on the chest, passes through the eye and ends on the nape. A
second band starts from the first few spines of the dorsal fin
and extends toward the pectoral fin base but is considerably
faded, almost absent, below the first few millimeters. There is
a distinct gap between these two bands. The question then
arises, how are these bands arranged in the Rio Negro angelfish?
It appears that they are similar in position to those of the
Orinoco angelfish. The difference in these two types of banding
is not all that great. All it takes to move from one to the
other is some melanophores to bridge the gap between the eye
band and the band from the first dorsal spines-which apparently
occurs in the Guyana populations and those from the Amazon. Not
enough wild-caught specimens are available to me to be able to
extrapolate further in the nuances of the different types of
head banding. It is interesting to note at this point that P. dumerilii goes one step further in that there is another “short”
band between the eye band and the band on the first dorsal fin
spines, the eye band itself simply crossing from one eye to the
other directly across the interorbital space.
Finally, there seems to be a more vertical appearance to the
Rio Orinoco individuals. Schultz considered the proportional
measurements as too variable and that comparison of specimens
even at “nearly equal sizes suggests that little reliance can be
placed on measurements for identification purposes.” The Orinoco
and Rio Negro populations show a tendency to be slightly deeper
bodied than the Amazon and Guyana populations in the limited
material at hand. Body depth of the former ranges from 1.2-1.3
in standard length whereas that of the latter ranges from
1.3-1.5 in standard length.
Repeating the question asked before, how many real
angelfishes are there? Answer-two, Pterophyllurn scalare and
P. dumerilii. But P. scalare is considered here to consist of two
subspecies, P. scalare scalare from the Amazon and Guyana and
P. scalare altum from the Orinoco and Rio Negro. These two
subspecies are separable on the basis of color pattern as
described above, a tendency towards higher meristic counts (at
least in the dorsal and anal fin soft rays and the lateral scale
rows) and perhaps a deeper body. P. dumerilii differs from
P. scalare in having lower counts in the dorsal and anal fin rays
and the lateral scale rows. These meristics are summarized in
the accompanying table.
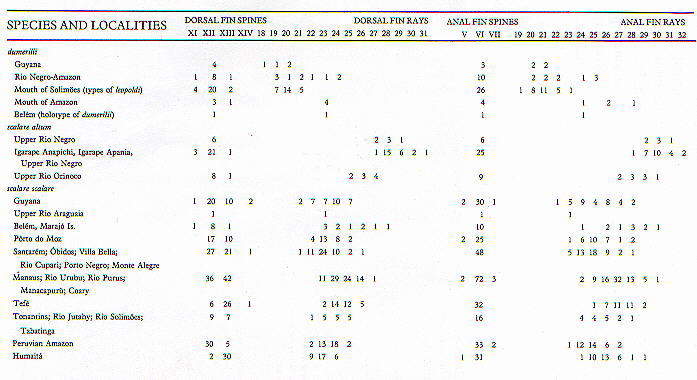
Fig.
2. Pterophyllum species
meristics sumerization.
|
| Fig.
2. Pterophyllum species
meristics sumerization. |
In addition, there are color pattern differences which can be
used for distinguishing P. scalare and P. dumerilii. In
P. dumerilii there is a conspicuous black spot located just below
the dorsal fin base between the two black bars. In P. scalare
there is a bar or the remnant of a bar in the same position, its
distinctness depending a great deal upon the mood of the
individual fish. As noted before, there is a separate bar
between the eye band bar and the one originating at the first
few dorsal fin spines. This would seem to be more similar in
appearance to P. scalare altum from the Orinoco and Rio Negro
and more distinct from the Guyana and Amazon populations. Since
P. dumerilii has only been found in the main Amazon and Guyana
regions with P. scalare scalare, they are more easily separated
by this character from the specimens found there.
As far as known, P. scalare scalare has a broad range over
the Amazon basin from the area of the mouth to Peru (Ucayali R.,
etc.) and from Guyana (Schultz also listed French Guiana in his
list of specimens examined) to the Araguaia River (almost 15 0S
latitude near Brasilia). Dr. Axelrod was able to extend the
range to the Madera River as far as Humaita.

Counts recorded for Pterophyllum species at various
localities in the Amazon basin and adjacent regions. Data from
Schultz, 1967 and new material.
|
| Counts recorded for Pterophyllum species at various
localities in the Amazon basin and adjacent regions. Data from
Schultz, 1967 and new material. |
P. scalare altum appears to be restricted to the area from
the Orinoco (at Atabapo) to the Rio Negro at least as far as
Igarape Anapichi and Igarape Apania. The Orinoco and Rio Negro
may have some connection through the Casiqulare River (where P.
s. altum has also been found).
P. dumerilii seems to be
restricted to the Amazon River from its mouth to Tonantins on
the Solimoes, as well as occurring in Guyana. Its distribution
seems to follow that of P. scalare scalare, although it is not
nearly as widespread or common. Perhaps as the species becomes
better known its distributional pattern will become clearer. |